|
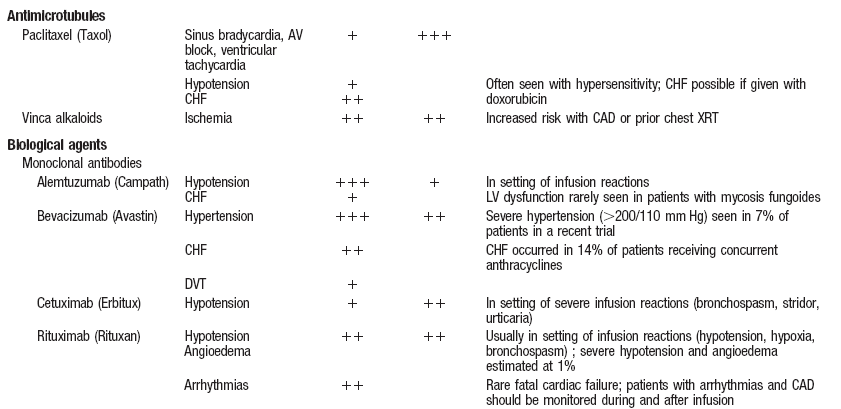
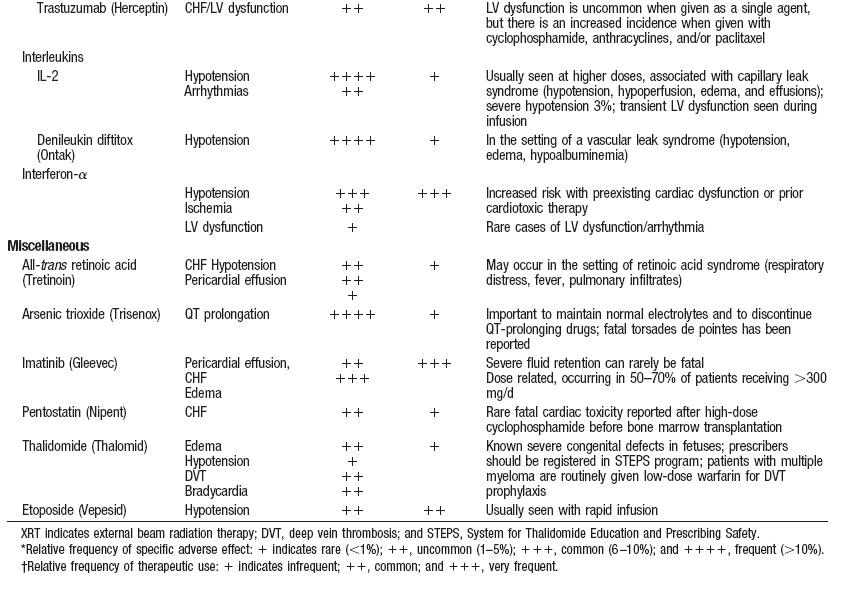
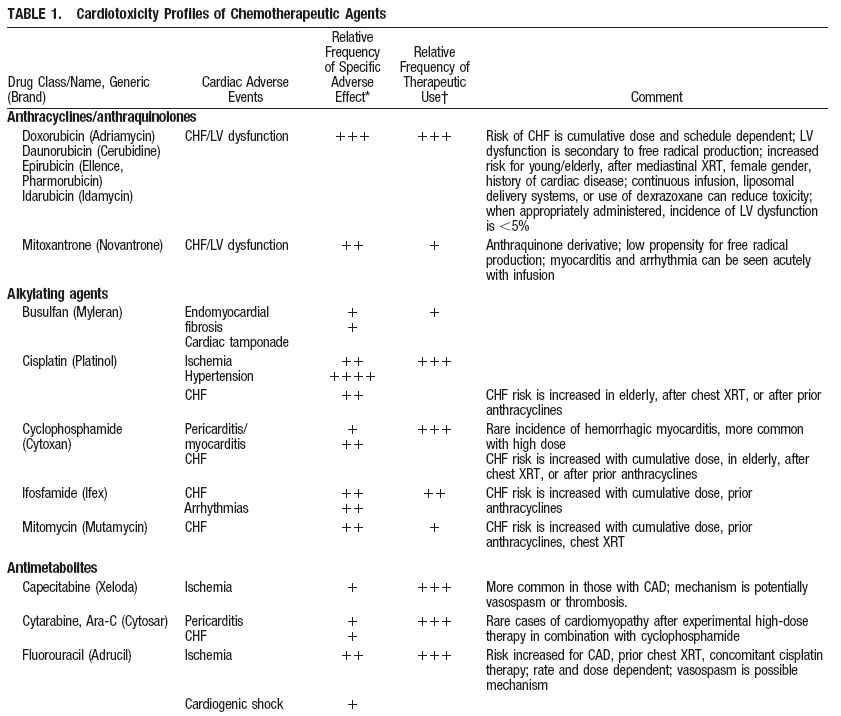
Cardiovascular Complications of Cancer Therapy.
Yeh ETH et al. Circulation. 2004; 109:3122-3131. |
|||
|
|||
 |
|||
|
|
Myocardial fibrosis is also a side effect of radiation therapy.83 Fibrosis is characterized by marked alterations in collagen synthesis.90 Valvular heart disease is also common after radiation because radiation causes fibrous thickening of cardiac valves.88 Left-sided valves are more often involved than right valves,83 and only a minority of patients with radiation-induced valvular disease have clinically moderate or severe dysfunction.88 The mean time from radiation to onset of symptoms is approximately 98 months.83
|
||
|
Monitoring Cardiovascular Toxicity The experience with anthracycline cardiotoxicity proved that the early detection and treatment of cardiotoxicity could significantly reduce the development of clinical manifestations. Endomyocardial biopsy is the most sensitive and specific way to diagnose and monitor anthracycline cardiotoxicity, 91 but the invasive nature of this procedure limits its use. Although guidelines have been developed for children receiving anthracyclines,92 no definite guidelines have been adopted for adults. The most common noninvasive method of monitoring myocardial toxicity from anthracyclines and other chemotherapeutic agents has been the assessment of LV systolic function, with either radionuclide ventriculography or echocardiography. Fractional shortening and LV ejection fraction are the most commonly used measurements,93 but both depend on preload and afterload. LV ejection fraction measurements also are not sensitive for the early detection of preclinical cardiac disease. Several studies have suggested that diastolic dysfunction is an early sign of anthracycline-induced cardiac dysfunction.94 Thus, measurements of diastolic function by Doppler echocardiography may be a sensitive method for early detection of toxicity.94,95 Provocative testing with exercise96 or dobutamine echocardiography97 has also been used to assess early anthracycline cardiotoxicity. Thus, these provocative-testing modalities may be sensitive for the early detection of subclinical cardiomyopathy and may provide an opportunity for therapeutic intervention before the development of overt LV dysfunction. Biomarkers such as troponin I and T98 may be useful in early detection of doxorubicin cardiotoxicity before the appearance of changes in LV ejection fraction, especially in children. During the past 10 years, several studies have confirmed the usefulness of B-type natriuretic peptide (BNP), a neurohormone elevated in response to volume overload, in the diagnosis and treatment of CHF.99 Recent studies in patients with cancer have shown that high levels of BNP correlated with impairment of LV function during anthracycline therapy.100,101 BNP has also been shown to be elevated before the development of LV dysfunction in patients undergoing high-dose therapy and hematopoietic stem cell transplantation.102 Monitoring for other anticancer drug–related cardiotoxic effects such as arrhythmias, ischemic cardiac events, and pericardial disease should be planned and specially tailored for each therapeutic protocol according to which anticancer agents are prescribed. Cardiac tests such as electrocardiography, rest and stress myocardial perfusion imaging, and troponin levels can be used to monitor ischemic cardiac complications. Twenty-four–hour Holter monitoring can be very helpful in detecting and evaluating suspected arrhythmias. Echocardiography has emerged as the test of choice for the noninvasive evaluation of cardiac disease as related to cancer therapy. This tool is essential in the evaluation of LV systolic and diastolic function, pericardial disease, and detailed evaluation of valvular heart disease. Doppler echocardiography can also be used to assess hemodynamic status, including the presence of pulmonary hypertension. In patients with chemotherapy-induced cardiomyopathy, a decrease in BNP levels after dobutamine stress echocardiography correlated with the presence of contractile reserve. This finding also correlated with long-term improvement in LV systolic function and New York Heart Association class rating when patients were given _-blockers and angiotensin converting enzyme (ACE) inhibitors.103 |
|||
|
Strategies to Reduce Cardiovascular Toxicity and Manage Complications Once cardiac toxicity is anticipated, strategies to control these adverse events can be developed, as evidenced by the changing patterns of anthracycline administration over time. Anthracycline toxicity can be minimized by reducing the total dose to <400 mg/m2 and changing the administration from a rapid infusion to a continuous infusion.104 Newer liposomal formulations also may reduce cardiotoxicity.105,106 Dexrazoxane, a derivative of EDTA, can reduce the amount of free iron in myocytes by producing free radicals that decrease oxidized iron levels during anthracycline infusion.107 Generally, dexrazoxane has been recommended for patients with metastatic breast cancer who have received cumulative anthracycline doses of >300 mg/m2; dexrazoxane is not recommended at the beginning of therapy because of the possibility of reducing the anticancer effect of the anthracyclines.108 Dexrazoxane has led to improved survival in some studies, but whether this improvement was due to improved cardiac status is unclear. What is known, however, is that dexrazoxane can worsen thrombocytopenia and granulocytopenia. The evolution of management strategies for trastuzumab-related cardiac toxicity is following a progression similar to that of the anthracyclines. After initial reports that the combination of trastuzumab, anthracyclines, and cyclophosphamide led to severe heart failure in up to 16% of patients with breast cancer undergoing this treatment, the administration strategy was changed to avoid giving these drugs simultaneously, and more stringent cardiac monitoring was instituted. These modifications have considerably reduced toxicity rates.48 Current studies of trastuzumab for a variety of breast cancer populations will further define the risk of LV dysfunction from such treatment. Trastuzumab-related cardiomyopathy seems to be largely reversible with appropriate therapy. Such therapy would include ceasing the drug, treating cardiac risk factors, and administering appropriate therapy for LV dysfunction.109 These principles apply to any cardiac toxicity discussed in this review but especially to the management of LV dysfunction. ACE inhibitors and beta-blockers are the cornerstones of therapy for LV dysfunction and should be administered to patients with cancer as aggressively as to any other patient population. Interestingly, rechallenge with trastuzumab does not necessarily lead to redevelopment of LV dysfunction or CHF,109 thus allowing important anticancer therapy to be continued without compromising the patient’s cardiac status. Much cardiac toxicity can be managed best by removing the offending agent. Unfortunately, in the case of newly developed LV dysfunction, chemotherapy may not be the only explanation for the reduced function, and thus all possible reversible causes should be investigated. In patients with cancer, ischemia is still a reversible cause of LV dysfunction. Cardiac reserve and subsequent improvement after aggressive CHF-based therapy can be predicted by results from dobutamine stress echocardiography.103 More importantly, once therapy is established, it may need to be continued because withdrawal of therapy in some patients has been associated with serious adverse events.110 |
|||
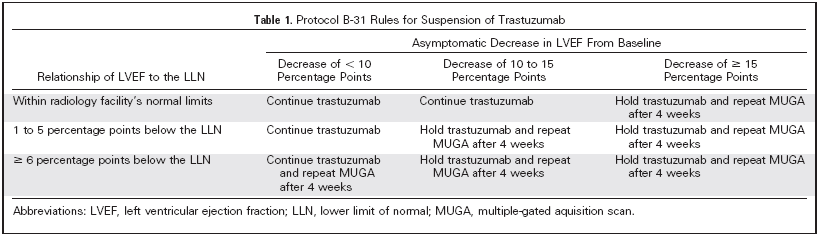 Priya Rastogi et al. J Clin Oncol 23:7811-7819. |
|||